 Hydrogen, symbol H in the periodic table, is the lightest and most abundant element in the universe.
Hydrogen, symbol H in the periodic table, is the lightest and most abundant element in the universe.
Hydrogen is a clean fuel because its combustion products are energy and water.
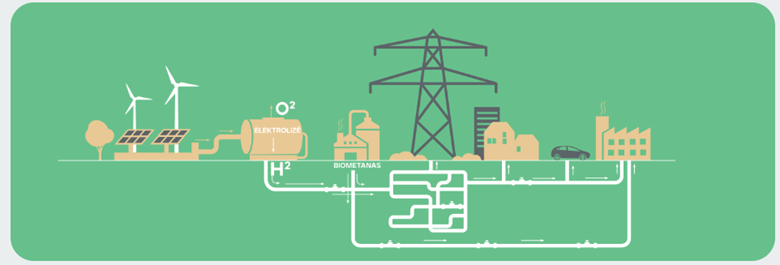
Electrolysis is one of the cleanest ways to produce hydrogen. It uses renewable electricity to split water into hydrogen and oxygen.
When hydrogen is produced using electricity from renewable sources such as wind or sun, CO2 emissions and air pollution are zero.
Hydrogen is odourless, tasteless, explosive, extremely flammable, most abundant chemical element, and accounts for 75% of the mass of the universe. Hydrogen boils at - 253°C. Hydrogen gas is not toxic, but it causes suffocation when inhaled. At just 4 percent concentration in the room, hydrogen gas can cause an explosion.
Depending on the raw materials and the method of production, hydrogen can come in different colours, including grey, blue, green, and more:
Black hydrogen is produced by gasification from coal.
Extraction from fossil fuel, or reforming, is the most common way of producing hydrogen by converting hydrocarbons and alcohols at temperatures between 700 and 9000 C. There are three types of reforming:
Green hydrogen is produced by electrolysis in so-called "power-to-gas" (P2G) plants. Such a plant is connected to renewable electricity generation facilities and the water supply system on one side and to the gas pipeline system on the other side. By passing an electric current through the water in a power-to-gas plant, water molecules are split into oxygen and hydrogen gas. Hydrogen gas is then injected into the pipeline network, where it mixes with natural gas and is delivered to consumers or stored in the pipeline.
For more information about hydrogen technology, click bellow:
Hydrogen can be transported by trucks, vessels and pipelines. When transported by land, gaseous hydrogen must be compressed to increase its energy density and to allow for a more efficient transportation. In the case of liquid hydrogen, highly insulated cryogenic tankers are used.
Hydrogen can be transported through the gas system (both transmission and distribution), as a mixture of natural gas and hydrogen, or by converting the gas system to transport pure hydrogen. Gas transmission system operators across Europe and other continents are upgrading their infrastructure to allow hydrogen or a mixture of hydrogen and natural gas to flow through pipelines.
Transporting hydrogen via pipelines over distances of several thousand kilometres is several times more cost-effective than by vehicles.
Our plan is to adapt the gas transmission system to the new energy in the next ten years, so that not only natural gas, but also hydrogen will be flowing through Lithuania's gas pipelines. It is important to identify the necessary gas system modifications to transport hydrogen to be performed. We are investigating the conditions for mixing hydrogen with natural gas and the behaviour of such a mixture in a pipeline. We are looking at the share which hydrogen could take up in the total gas flow, and the impact of a hydrogen-gas mix on the entire gas system, both transmission and distribution. The economics are also very important, with the need to calculate how much hydrogen will be consumed in the short and long term.
Hydrogen storage is the field that has not been studied extensively. In order to assess the opportunities and benefits, it is necessary to carry out a feasibility study on geological storage of hydrogen, above-ground storage of ammonia, methanol and to assess the potential for hydrogen storage on a regional scale, i.e. what could be the potential for accessing hydrogen storages of other countries in the region.
In addition, the potential of synthetic methane as a long-term hydrogen storage option needs to be explored. The production of synthetic methane from green hydrogen would allow storage to balance seasonal fluctuations in demand and maintain the existing natural gas infrastructure. The analyses carried out show that ammonia can also play an important role in the development of Lithuania's hydrogen sector, thus opportunities for its storage must be properly assessed.
Hydrogen can replace fossil fuels in some polluting industrial processes, reduce GHG emissions and strengthen the future competitiveness of chemicals, metals and other polluting industrial sectors. Hydrogen can be used as a raw material, a fuel, an energy carrier or an energy storage medium. It also has a wide range of applications in the industrial, transport and energy sectors. In order to reduce dependence on fossil fuels, hydrogen can be used as a feedstock or as an energy source in processes and sectors where direct electrification is technically not feasible or uncompetitive. Its potential is also seen in the energy sector as a mean of balancing the energy system and storing surplus RES energy.
Industry. In the industrial sector, hydrogen plays an important role in the production of a wide range of chemical compounds, purification of petroleum products and the processing of metals. Hydrogen is used as a raw material in the production of ammonia, methanol, hydrogen peroxide, solvents, plastics, polyester and nylon. Hydrogen gas is used in furnaces to harden metals, while hydrogen and oxygen flames are used to cut ferrous metals. Hydrogen is also often mixed with argon and used to weld metals.
Transport. In the transport sector, hydrogen gas can be used as a non-polluting fuel alternative to petrol and diesel. Hydrogen in gaseous or liquid form can be used in fuel cells or in specially adapted internal combustion engines, and its combustion does not release harmful particles into the environment. Hydrogen has huge potential as an alternative fuel in the transport sector. Some existing vehicles, in particular cars, can be replaced by electric vehicles, but the electrification of heavy vehicles is much more difficult due to limited technical feasibility and high costs. The use of batteries in heavy transport poses practical application problems, and other energy sources (such as hydrogen, synthetic methane or ammonia) may be a better alternative to provide fuel for trains on non-electrified lines, freight ships or airplanes.
Energy. In the energy sector, the development of RES electricity generation capacity is necessary to reduce dependence on fossil fuels. The dependence on climatic weather conditions makes it difficult for RES generation facilities to ensure the stability of electricity generation, which complicates electricity transmission and distribution. In order to make efficient use of the electricity generated and to reduce the technical challenges associated with grid management, it is necessary to develop energy storage solutions that allow the storage of surplus energy to be used when there is a shortage of electricity on the market. The production of green hydrogen by electrolysis could be exploited for power grid flexibility services and assurance of stabilisation while implementing hydrogen storage solutions. As hydrogen can be used to store large amounts of energy for long periods of time, hydrogen production and storage technologies have the potential to compensate for seasonal fluctuations in electricity demand. Moreover, hydrogen can be transported by trucks, vessels or via a pipeline, so RES electricity can be generated where it is most efficient and transported over long distances in the form of hydrogen, without putting a strain on the power grid. Hydrogen can also be converted into value-added products used in various sectors, such as synthetic methane, ammonia, etc.
Power-to-X. Power-to-Gas (P2G) is the process of converting surplus RES (RES surplus is periods of time when RES generation is high and the market price of electricity is low) into hydrogen gas using electrolysis (a method of separating conjugated chemical elements and compounds by passing an electric current through them). Hydrogen can be used directly or further steps (known as two-step P2G systems) can convert the hydrogen into synthetic gas, methane or liquefied hydrocarbon gas. This process is also known as Power-to-X (PtX), where X refers to any product made from hydrogen gas that has been generated from surplus RES.
Fertilisers and oil refining sectors. The fertiliser industry currently has the highest demand for hydrogen, but the price of hydrogen poses a major risk to the competitiveness of this sector.
In addition to the fertiliser industry, green hydrogen is used in the oil refining sector and could potentially be used in other high-temperature industrial processes to replace natural gas. The potential for hydrogen production and use in industry in Lithuania is also linked to economic activities where processes require extremely high and stable temperatures (glass, cement production, etc.).
Household. Hydrogen can be used in households. It allows energy to be stored in the summer and used in the winter: when unused energy is stored in a battery and, once the battery is full, hydrogen is produced for the winter. In the cold season, the stored hydrogen is used to generate electricity and charge the battery. The heat generated by electrolysis can be used to produce hot water in the summer and to heat premises in the winter. The first 300 houses equipped with boilers, heaters and cooking appliances that use hydrogen gas were presented in Scotland.